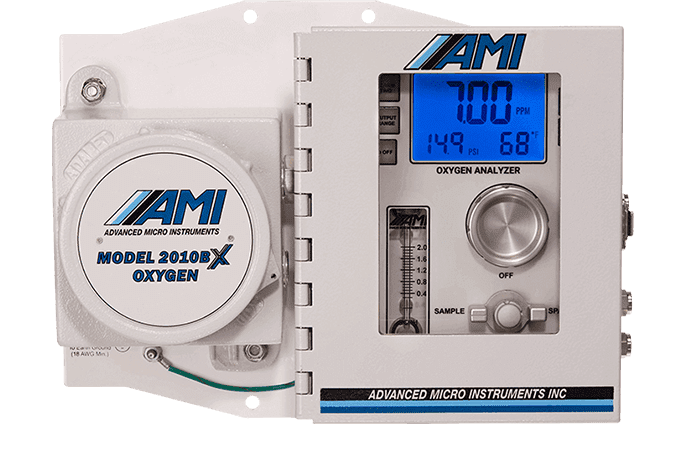
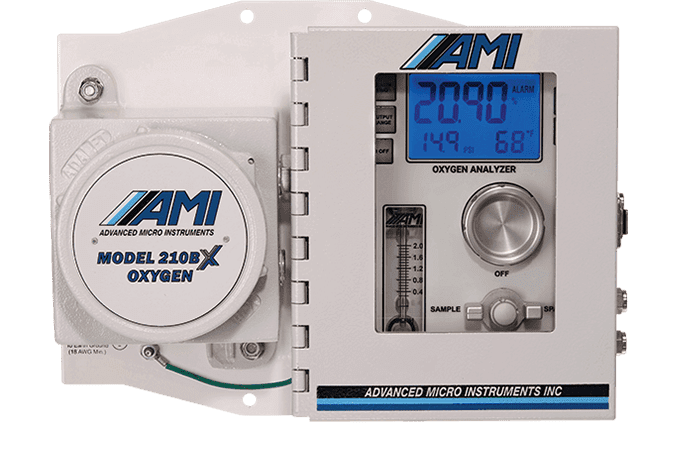
Why Measure Oxygen?
Oxygen is the 3rd most abundant element by mass in the universe. While critical for life, its presence or absence, as well as its levels, plays a crucial role in many industrial processes.
In gas plants, transmission lines and gathering systems, the presence of even trace levels of oxygen can pose serious problems. It indicates that a leak has developed in the infrastructure and, if left unsealed, can lead to further costly damage and halt the movement of natural gas.
In other industrial processes, keeping oxygen concentrations within predetermined levels is crucial for manufacturing. Companies, including those in aerospace, air separation and electronics, introduce nitrogen gas as part of their production to bring oxygen levels into acceptable levels. Thus, they need a way to monitor those percent levels.
Last, monitoring oxygen levels is important for some companies because of safety. Laboratories or environments that handle pressurized gas cylinders or dewers that contain cryogenically liquefied gases, must be diligent about detecting leaks. A leak from any of these containers can quickly displace oxygen levels within an enclosed space and move them below dangerous thresholds. This puts their personnel in the vicinity at risk for not only oxygen depletion but also for death by asphyxiation. OSHA has published guidelines that establish safe levels for oxygen in the workplace.
Oxygen Measurement Technologies
There are multiple technologies available for the detection and ongoing monitoring of oxygen levels in gas streams, industrial processes, or controlled environments. The reliability and accuracy of the oxygen readings are critical for technicians to make real-time decisions on their operations. Each measurement technology may be more desirable in specific applications and all have some limitations. Here are a few of the most common ones:
Zirconium-oxide | Zirconia becomes conductive to oxygen when it is held at high temperatures. Sensors constructed from zirconium oxide are commonly used oxygen analyzers to provide a reliable and robust method for measuring oxygen in percent levels. The sensors must be kept at an elevated temperature - up to 700° C - to function correctly. | Pros:
| Cons:
|
Electrochemical | An electrochemical sensor functions much like a battery consisting of a cathode, and anode and an electrolyte. The sensor will produce a current output proportional to the oxygen partial pressure in the surrounding gas. When no oxygen is present an electrochemical sensor will read a true zero meaning analyzers using this technology can measure trace oxygen levels in the parts per billion range. | Pros:
| Cons:
|
Paramagnetic | Paramagnetic technologies take advantage of the natural attraction of oxygen molecules to strong magnetic fields. This approach involves a sampling system where nitrogen filled spheres are suspended within a strong magnetic field. As oxygen in a gas passes through the sample area, it is attracted to the magnetic fields, creating a force on the spheres. The movement of the spheres, which is proportional to the oxygen concentration, is measured and converted to an oxygen concentration. | Pros:
| Cons:
|
Optical Fluorescence Quenching | Optical type oxygen analyzers such as fluorescence quenching are capable of providing trace measurements of oxygen in gas streams. They utilize an optical fiber coated with chemical that will luminesce when it is excited by a laser light. The oxygen content in the gas stream will proportionally counteract the fluorescence of the chemical. This is measured using an optical sensor. | Pros:
| Cons:
|
Selecting an Oxygen Analyzer
Step 1: Select Measurement Capability
The first step will be choosing the measurement range of oxygen you will want to monitor in your application
Step 2: Select Analyzer Configuration
The second step will be to decide the appropriate style of analyzer based on its intended application and use
Measurement Capability
Analyzer Configuration
Analyzer Design – Things to Look For
There are many different Oxygen Analyzers on the market, made and sold by different companies. Though each one touts the benefits of the underlying measurement technology, it is important for the buyer to evaluate other keys areas of the design.
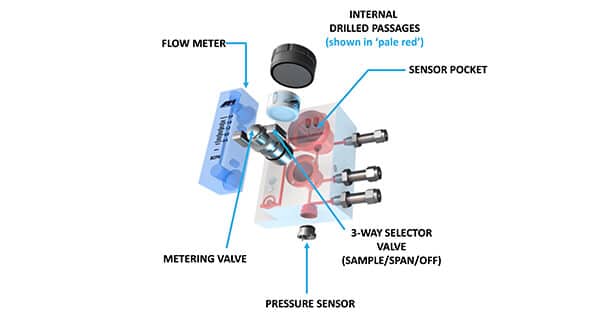
Sample Handling Components
For the sample gas to move from its source to the location inside the Analyzer for a reading, critical sample handling components must be installed along the sample gas path. Among these are the flow meter, metering valve, tubing and selector valve for gas flow. Some oxygen analyzers even include additional items, such as a pressure sensor and temperature transducer. Any customer considering a purchase should ask these questions: “Does this Analyzer have everything that I need? Or do I have to purchase additional components to install with my Analyzer before I can start taking measurements?”
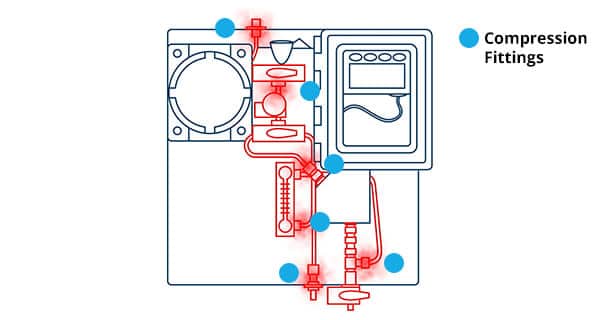
compression fittings are prone to leaks.
Susceptibility of Leaks in the Gas Path
Moving sample gas to its target destination requires setting up a pathway with critical sample handling components connected to it. Many manufacturers, as well as customers, turn to using long lengths of stainless-steel tubing with compression fittings to make this happen. Though the compression fittings can connect the sample handling components to the stainless-steel tubing, they are prone to leaks over time and can affect the performance of the Analyzer.
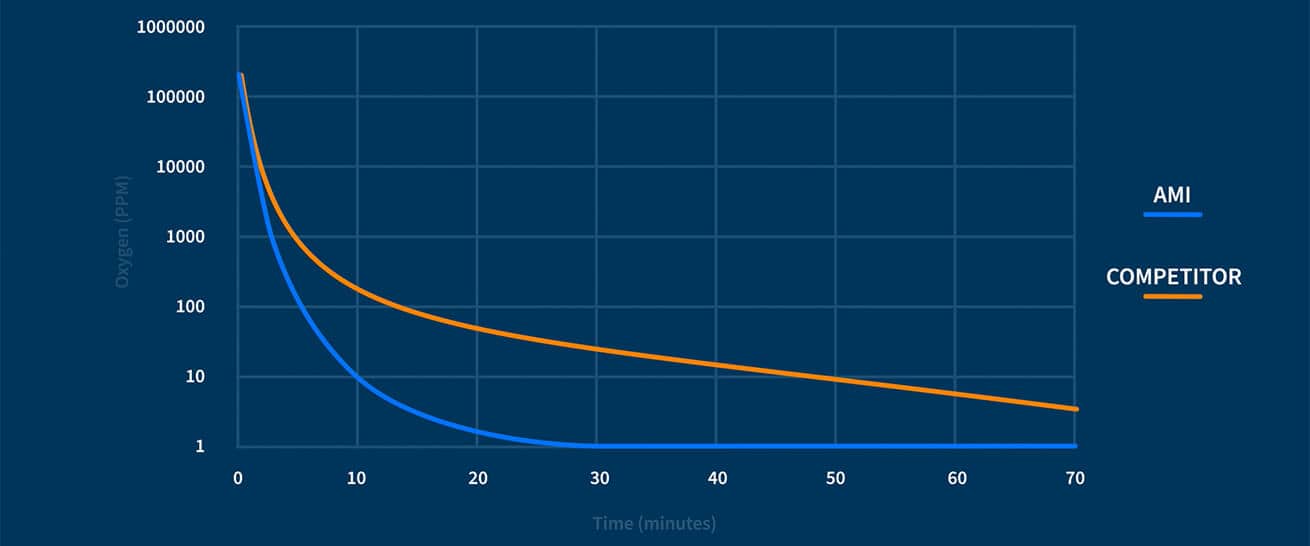
This is particularly serious for trace measurements because even the smallest levels of oxygen entering the system will impair the accuracy and reliability of the Analyzer’s readings.
Therefore, be sure to consider the reliance of an analyzer design on compression fittings for its sample gas path.
a much shorter gas path.
extends the gas path.
Length of the Gas Path
The Analyzer’s measurement response time reflects how fast the unit can deliver a reading. One of the design parameters that affects this is the length of the sample gas path. A shorter gas path delivers a faster reading, while a longer one does the opposite.
Because the actual gas path can consist of several turns and detours prior to reaching the site of where the measurement takes place, it is generally not ideal to rely solely on the dimensions of the Analyzer. Instead, contact the manufacturer and ask how far it takes the sample to travel inside the Analyzer before a reading can take place.
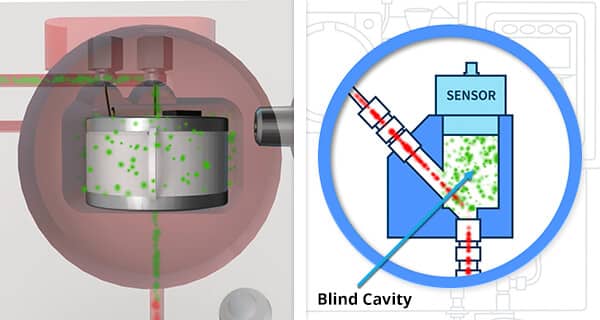
Flow Efficiency of the Sensor Pocket (for Analyzers utilizing sensors)
The other parameter of an analyzer design that affects the response time is the flow efficiency of the sensor pocket. A good design will allow a rapid and full purge of the sensor pocket. This will allow the sensor to respond quickly and detect any changes in the sample gas. In contrast, a poorly designed pocket can have a ‘blind cavity’ that limits the exposure of the sensor to the sample gas and create ‘dead legs’ which slows the exit of ‘previous sample gas’ from the sensor area. All of which slows the Analyzer from delivering a measurement that accurately reflects the sample gas in ‘real-time’.
Anyone buying an Oxygen Analyzer utilizing sensors should evaluate how well the sensor pocket is designed.
Sample Conditioning & Maintenance

the sample gas prior to it entering the Analyzer.
To help oxygen measurements remain accurate and reliable, users should be cognizant of proper sample conditioning practices. First, care should be taken to avoid getting liquid into the oxygen analyzer, which could come from hot or wet gas, as well as, the occasional moisture slugs from the pipeline. Operators should install devices that bring hot sample gas to ambient temperature to avoid condensation. They also should install liquid rejection instruments, capable of stopping larger slugs of fluids from flooding the Analyzer. Second, it is important to regulate the sample gas down to the recommended operating pressure of the Analyzer to avoid stressing the internal components beyond their specified limits. Proper sample conditioning not only maintains the performance of the Analyzer but extends its operational life.

for easy replacement.
Most Oxygen Analyzers require periodic maintenance, and the work required to complete it varies from manufacturer to manufacturer. Some need the user to only replace the electrochemical sensor, while others require a cleaning to remove accumulated dirt and dust from the sensing element. Because the procedure(s) can be easy or hard to do, as well as time-consuming, anyone interested in purchasing an Oxygen Analyzer should weigh the maintenance in the purchase decision.